DESCRIPTION
SPECIFICATION
RESOURCES
Service Description
BGI provides LC-MS/MS peptide mapping services to support biopharmaceutical and biotechnology applications. Our Peptide Mapping services are designed to characterize and monitor the molecular details of a therapeutic protein drug at each position in the amino acid sequence1,2. Meanwhile, Disulfide Mapping and Epitope Mapping services are provided as well.
Project Workflow
Sequence Verification
We provide amino acid sequence verification services and PTM mapping for purified proteins. Our service is useful for accurately validating expression of recombinant proteins or identifying process related PTMs on biologic drugs.
- Gentle sample preparation
- Individual samples
- Peptide map data analysis
Molecular Assessment
We can help you characterize amino acid sequence liability. We provide a Molecular Assessment service to monitor specific amino acid modifications in response to a forced degradation treatment customized to meet your project needs. Our service is optimized for high dynamic range peptide sequence coverage.
- Forced sample degradation series
- Gentle sample preparation
- LC-UV-MS/MS DDA Analysis
- Peptide map data analysis
- Molecular Assessment Profile
References
[1]. Lauber MA, Koza SM, McCall SA, Alden BA, Iraneta PC, Fountain KJ. High-resolution peptide mapping separations with MS-friendly mobile phases and charge-surface-modified C18. Anal Chem. 2013 Jul 16;85(14):6936-44. doi: 10.1021/ac401481z.
[2]. Mouchahoir T, Schiel JE. Development of an LC-MS/MS peptide mapping protocol for the NISTmAb. Anal Bioanal Chem. 2018 Mar;410(8):2111-2126. doi: 10.1007/s00216-018-0848-6.
How to order
Mass Spectrometry Service Specification
Peptide Mapping services are performed using analytical flow liquid chromatography, UV detection, and high resolution Orbitrap mass spectrometry.
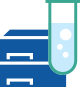
Sample Preparation and Services
- Gentle digestion performed using sequencing-grade trypsin or alternative proteolysis designed to minimize sample preparation artifacts
- In-line UV detection also possible. Optimized for minimal sample oxidation
- Each fraction analyzed using 150 min analytical flow LC-MS/MS using a Q Exactive HF-X Orbitrap mass spectrometer
- Data analysis and validation performed with BioPharma Finder software
- We can work with you to develop customized Multi-Attribute Method (MAM) services using Chromeleon 7 software (21 CFR Part 11 compliant)

Quality Standard
- Summary includes all methods and data analysis
- Reports provided in Excel or PDF format, RAW files available upon request

Turn Around Time
- Typical 20 working days from sample QC acceptance to data report delivery
Sample Requirements
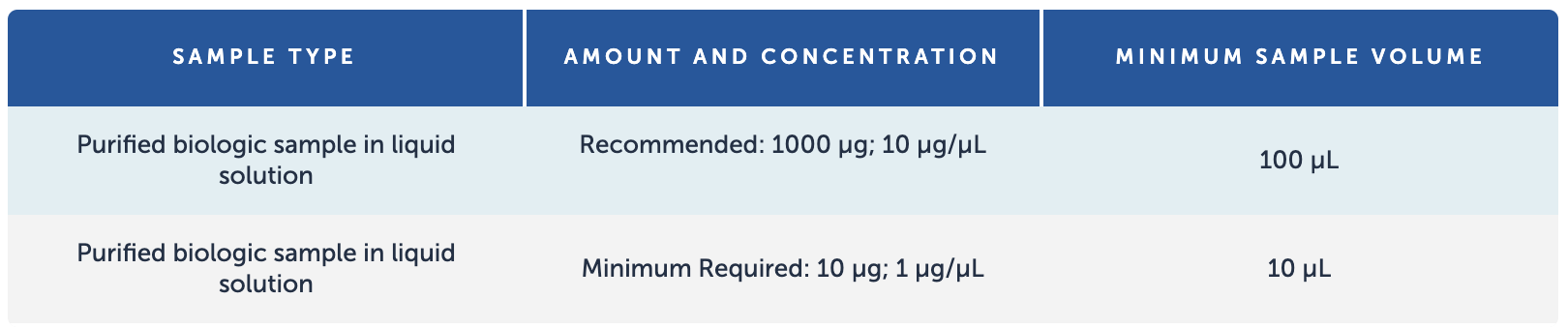
Data Analysis
ADVANCED BIOINFORMATICS
- Data analysis and validation performed with BioPharma Finder software
- Peptide mapping method for identifying sequence variants, unknown modification, and PTM co-occupancy
- Amino acid site PTM status and relative abundance
- Reports provided in Excel or PDF format, RAW files available upon request

