Description
Workflow
Resources
Introduction
BGI is changing diagnostics by providing fast, affordable and comprehensive genetic knowledge.
Genetic disease raises many questions. We’re empowering healthcare providers with the information they need to answer these questions, in order to both prevent disease and better guide patient treatment decisions.
With more than 5 years’ experience in diagnostic testing and screening, and a worldwide network of offices and laboratories, BGI acts as one of the world’s most tried and trusted clinical genomics partners.
A Trusted Local Solution for Non-invasive Prenatal Testing
Non-invasive prenatal testing (NIPT) has been proven superior to traditional screening methods for detecting common fetal aneuploidies. By reducing false positive rates, NIPT helps limit invasive procedures—and therefore, the risk of miscarriage—when used as a primary screen.
BGI has many years experience providing the NIFTY® Test to partners across the world, and has processed more than 3 million samples to date. As a trusted NIPT provider, we are proud to offer the next step in the evolution of NIPT testing by offering a local laboratory solution powered by our own proprietary sequencing platform, the BGISEQ-500.
Our Technology
BGISEQ-500 is an industry leading high-throughput sequencing solution, powered by combinatorial Probe-Anchor Synthesis (cPAS) and improved DNA Nanoballs (DNB) technology. The cPAS chemistry works by incorporating a fluorescent probe to a DNA anchor on the DNB, followed by high-resolution digital imaging. This combination of linear amplification and DNB technology reduces the error rate while enhancing the signal. In addition, the size of the DNB is controlled in such a way that only one DNB is bound per active site. This patterned array technology not only provides sequencing accuracy, but it also increases the chip utilization and sample density.
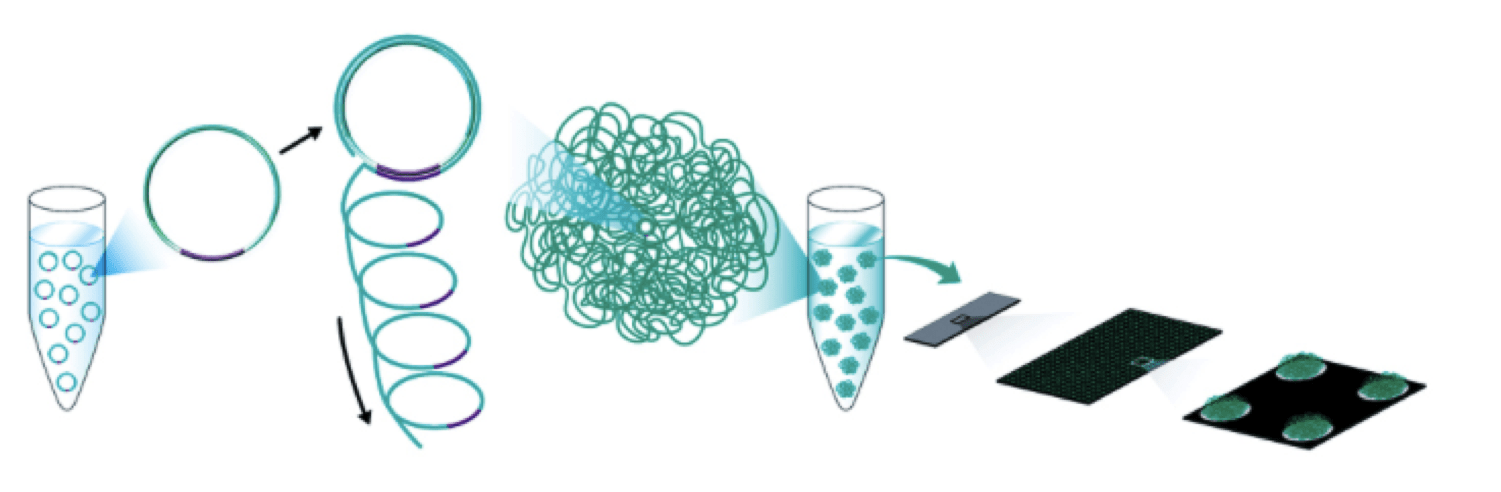
Notes:
The NIFTY® Local Lab Solution is an in vitro diagnostic test intended for use as a sequencing-based screening test for the detection of fetal aneuploidies from maternal peripheral whole blood samples in pregnant women of at least 10 weeks gestation. The test provides information regarding aneuploidy status for chromosomes 21, 18, 13, X, and Y. This product must NOT be used as the sole basis for diagnosis or other pregnancy management decisions.
Noninvasive prenatal testing (NIPT) based on cell-free DNA analysis from maternal blood is a screening test; it is not diagnostic. Test results must not be used as the sole basis for diagnosis. Further confirmatory testing is necessary prior to making any irreversible pregnancy decision.
Our local laboratory solution is not available in all countries or regions. Contact us for regional availability.
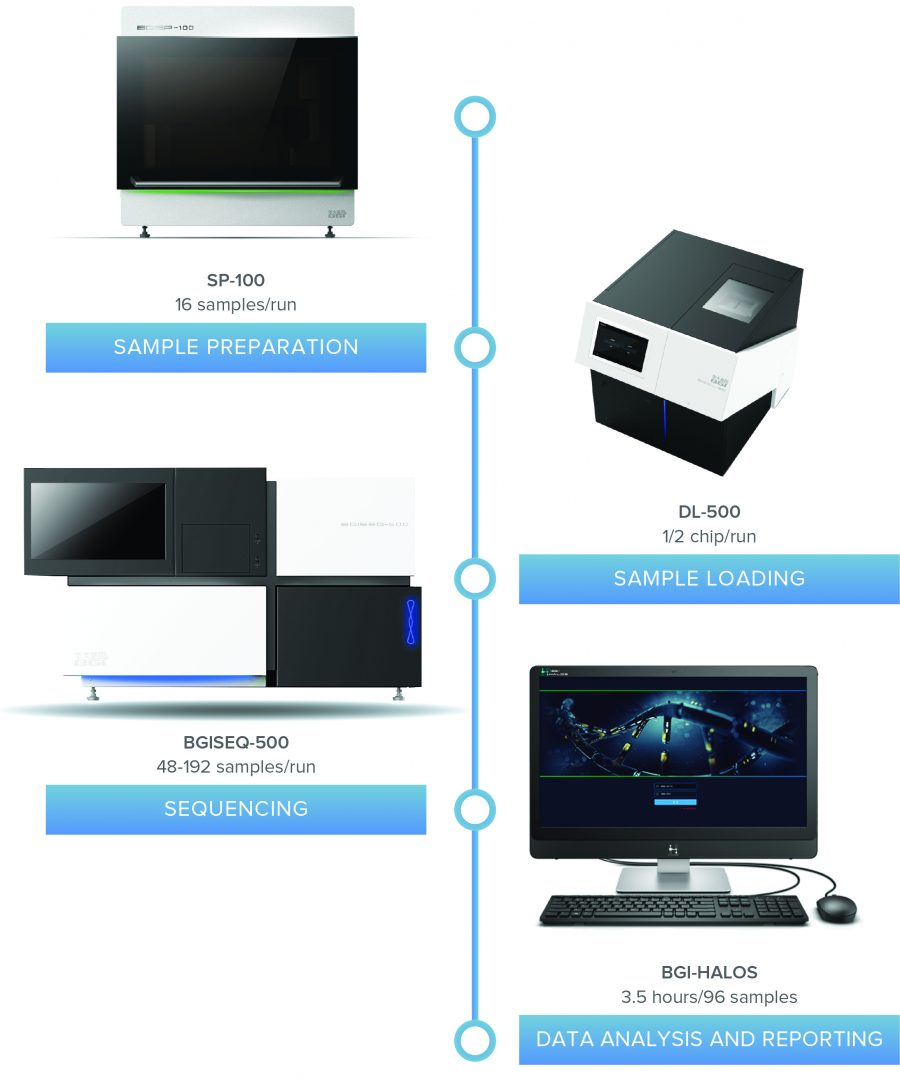
One Comprehensive Workflow
The NIFTY® Local Lab Solution is an automated and validated workflow including CE-IVD–marked sample preparation and assay software. The solution provides results for up to 96 samples in under 3 days. From sample prep to sequencing to analysis, the NIFTY® Local Lab Solution streamlines your process as one comprehensive workflow.
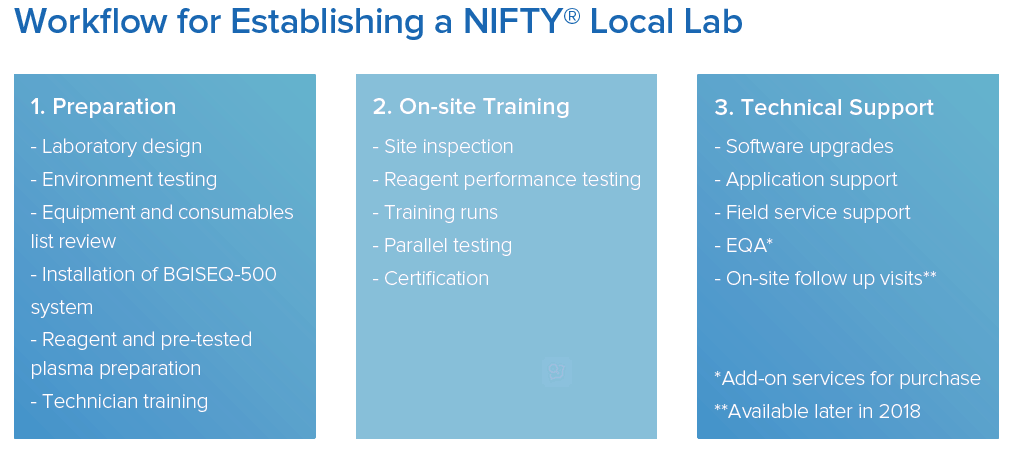
Establishing a Laboratory
BGI has developed an established process designed to ensure the efficient and economical set-up of your laboratory. Our dedicated and professional team will guide you through the entire process, from laboratory design to active service.
Reagent Solutions
BGI produces specially designed kits for full preparation of samples. Kits are CE IVD certified. By producing kits itself, rather than relying on external suppliers, BGI can ensure quality and control costs, passing on savings to our customers.

Learn More
Learn more about our laboratory solution by downloading our brochure or contact a BGI representative for more information by using the contact form on this website.
