RT-PCR Kit FAQs
Frequently Asked Questions (FAQs)
BGI Real-Time Fluorescent RT-PCR Kit for Detecting SARS-CoV-2 and US FDA Emergency Use Authorization (EUA) workflow components.
1) What instruments, consumables and reagents are required for running your RT-PCR assay? Where can I find the full list of EUA products and protocols?
Go to https://www.bgi.com/us/bgi-covid-19-total-lab-solution/ to see the integrated workflow outlining the instruments and reagents authorized by the FDA to run our assay. The full list of EUA products and protocols can be found from our Instructions for Use at: https://www.fda.gov/media/136472/download.
2) What real-time PCR systems are approved under the EUA?
- Applied Biosystems 7500 Fast
- Applied Biosystems 7500
- Applied Biosystems QuantStudio 5
- Roche LightCycler 480 Instrument II
3) What specimen types can be tested with your kit?
Our kit is currently approved for specimen types as follows:
- Throat (oropharyngeal) swabs
- Nasopharyngeal swabs
- Anterior nasal swabs
- Mid-turbinate nasal swabs
- Nasal washes
- Nasal aspirates
- Bronchoalveolar lavage fluid (BALF)
4) What comes in the BGI Real-Time Fluorescent RT-PCR Kit for Detecting SARS-CoV-2? Can I buy some of the vials in your kit?
The kit consists of four vials required for SARS-CoV-2 detection:
- SARS-CoV-2 Reaction Mix
- SARS-CoV-2 Enzyme Mix
- SARS-CoV-2 Positive Control
- SARS-CoV-2 No Template Control
Customers need to order the whole kit during transactions. We are unable to sell some of the vials in the kit.
5) What are the advantages of your RT-PCR test over other tests on the market?
- Clinically tested – Kit validated with large COVID-19 patient cohort
- Highly sensitive – Detect as low as 100 viral copies/mL for BALF samples
- Highly specific – No cross-reactivity with 54 human respiratory pathogens
- Fast turnaround – Sample to result in 4 hours with automated sample preparation system
- High-throughput – Ramp up labs for large-scale, community-based testing
- Ease of use – All inclusive with pre-mixed reaction reagents and controls
- Easy interpretation – Analysis of one target with well-defined controls
6) I learned that your kit received an EUA amendment from the FDA in April 2020 after receiving an EUA in March 2020. Can you tell me more about this?
The EUA amendment (April 2020) expands the original EUA label (March 2020) and further authorizes the use of the following for SARS-CoV-2 detection by the BGI RT-PCR kit: 1) MGISP-960RS automated sample preparation system (software v1.2), 2) MGIEasy Nucleic Acid Extraction Kit for viral RNA extraction, 3) Five additional human respiratory samples, and 4) Addition of three real-time PCR systems. This total lab solution addresses major bottlenecks in COVID-19 testing by offering high-throughput testing capability, broader clinical adaptability, and abundant supply of MGIEasy Nucleic Acid Extraction Kit.
EUA amendment: https://www.fda.gov/media/137354/download
Original EUA: https://www.fda.gov/media/136473/download
7) What gene target is used in your assay?
ORF1ab gene of SARS-CoV-2.
8) What is the Limit of Detection (LoD) of your test for detecting SARS-CoV-2?
The LoD for throat swab is 150 viral copies/mL. For BALF, the LoD is 100 viral copies/mL.
9) What is the turnaround time of your test when the MGISP-960RS automated sample preparation system is used?
The whole workflow for the test would take 3.25 hours from sample to result: automated RNA extraction and PCR master mix preparation for 36 to 96 samples (60 min), RT-PCR setup (15 min), running PCR and performing data analysis (2 hours). This workflow would take approximately 3.68 hours if the number of samples increases to 192.
10) What is the turnaround time of your test when the MGISP-960RS automated sample preparation system is not used?
The whole workflow for the test would take about 3 hours from sample to result if sample preparation is handled manually: RNA extraction and PCR master mix preparation (30-45 min), RT-PCR setup (15 min), running PCR and performing data analysis (2 hours).
11) Does your test include an internal control, and if so, what is it?
Yes. An internal reference control is included. This control targets human beta-actin gene.
12) Is positive control provided in your kit?
Yes. A positive control is provided in the kit. This control contains “pseudo-virus” or virus-like particle in which the synthetic viral RNA target and human beta-actin target in protein are coated separately.
13) What extraction control shall I use with the assay?
The positive control provided in the kit can serve as an extraction control.
14) Which viral RNA extraction methods are recommended for your kit?
MGIEasy Nucleic Acid Extraction Kit (MGI, catalog# 1000020261 or 1000020471) or QIAamp Viral RNA Mini Kit (Qiagen, catalog# 52904 or 52906).
15) How many controls should I use for each experiment?
At least two controls: one positive control and one no template control should be run in each experiment.
16) What is the principal design of the assay?
The assay uses Taqman probes that are designed to anneal to the target sequences. ORF1ab gene of SARS-CoV-2 and human beta-actin will be amplified by primers. The oligonucleotide probes are dual-labeled with a FAM fluorophore attached at the 5’ end and a quencher at the 3’ end. When the fluorophore and the quencher are in proximity, fluorescence signals are inhibited. During PCR extension, the 5’ to 3’ exonuclease activity of Taq polymerase will cleave the probes that have annealed to their target regions. The released fluorophore from the probes are now away and relieved from quenching by the quencher, resulting in fluorescence signals. The FAM channel of the real-time PCR machine is used to detect the ORF1ab gene of SARS-CoV-2 while the VIC/HEX channel is used for detecting the human beta-actin gene.
17) How do I interpret the control results?
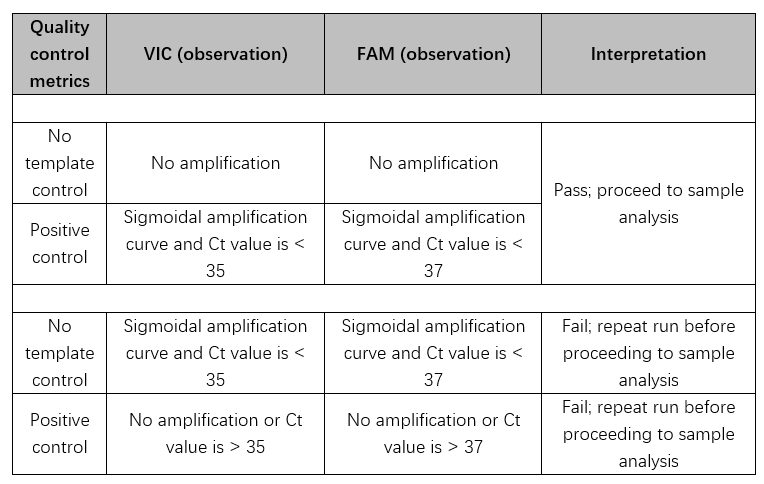
18) What Ct cutoff values should be used when interpreting the assay data?
Ct cutoff of 37 should be used for the FAM channel. For the VIC channel, Ct cutoff of 35 should be used.
19) Do you have data for the clinical performance of your test?
Yes. Our kit has been clinically validated. The Instructions for Use (IFU) provides the performance data in details. IFU: https://www.fda.gov/media/136472/download.
20) Are there any independent studies that demonstrate the clinical performance of your kit?
Yes. Researchers from the 1) RIVM, the Dutch National Institute for Public Health and the Environment, 2) Foundation For Innovative New Diagnostics (FIND), a WHO Collaborating Centre for Laboratory Strengthening and Diagnostic Technology Evaluation, and 3) Sinai Health System/University of Toronto, have all demonstrated that the BGI kit outperforms the other kits on market with respect to the limit of detection, making our kit particularly useful for detecting samples of low viral loads.
RIVM: https://www.sciencedirect.com/science/article/pii/S1386653220301542
FIND: https://www.finddx.org/covid-19/sarscov2-eval-molecular/molecular-eval-results/
Sinai Health: https://www.biorxiv.org/content/10.1101/2020.05.12.092387v1
21) How does your detection kit inventory look like in US and Canada?
BGI stocks the detection kit at our local warehouses in US and Canada, respectively. We frequently replenish our inventory to meet our customer demand. In addition, the kit is manufactured in our facility with manufacturing capacity of 2 million tests per day.
22) At what temperature is the kit maintained during shipping?
The kit is shipped with dry ice where the temperature is maintained at approximately -80ºC.
23) What emergency use authorizations or regulatory approvals did the kit receive?
In 2020, our kit received emergency use authorizations or regulatory approvals around the world:
- Jan 26: Emergency approval from China’s National Medical Products Administration (NMPA)
- Feb 25: CE-IVD Mark
- Mar 26: Emergency Use Authorization (EUA) from US FDA
- Mar 27: Emergency approval from Japan’s PMDA
- Apr 10: Australian Register of Therapeutic Goods from Australia’s TGA
- Apr 24: EUA Amendment from US FDA
- Apr 27: Provisional Authorization from Singapore’s Health Sciences Authority
- May 4: Importation and Sale Authorization under Interim Order from Health Canada
- May 7: WHO Emergency Use Listing for IVD detecting SARS-CoV-2
* Download the FAQ (PDF document).